Research Interests
The site-specific modification of proteins and enzymes by designed organic compounds is an area of growing importance. We have developed new methods to modify the oxygen-carrier protein, hemoglobin, so it can be used for a number of applications. For example, we have produced multifunctional reagents that cross-link hemoglobin at specific sites, introducing properties that make the material suitable to be used as an alternative for red cells in blood transfusions. We extended the method to include ways to combine cross-linking within a protein and connecting with a second (or additional) protein. This permits the interactions of assembled proteins to be studied in a defined system. We recently have improved the process by applying the CuAAC click procedure that we adapted specifically for coupling of two cross-linked hemoglobin-azides with bis-alkynes.
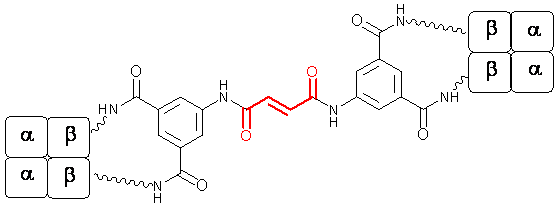
Connecting and cross-linking two hemoglobins
Thiamin diphosphate-dependent decarboxylases
Synthetic intermediates are lack the reactivity of the enzymic systems in the normal reaction. They also divert the reaction to destroy the cofactor. Enzymes that have related intermediates prevent decomposition. We are studying the detailed mechanism of these processes. The critical step is a very rapid breaking of a carbon-nitrogen bond, competing with a proton transfer as a rate-determining process.
upper route is fast and leads to destruction of thiamin.
lower route is slow - accelerated by enzyme
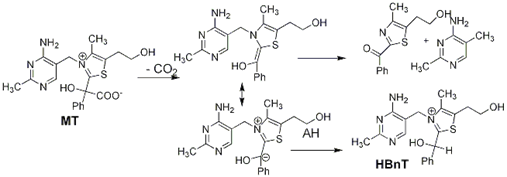
Decarboxylation mechanisms
Reversibility
The replacement of a carboxyl group by a proton appears to be a simple matter of forming CO2 by breaking a C-C bond. However, catalytic and isotope effect patterns remind us that the CO2 is a powerful electrophile, making the process easily reversible prior to the separation of the CO2 molecule from the residual carbanion. We are examining the modes by which this process can be accelerated despite the reversibility. We find that enzymes may function by enhancing the "forward commitment", which can be diagnosed by an increased 12C/13C kinetic isotope effect. We also have found that in some cases decarboxylation is accelerated in acidic solutions. We are examining related reactions involving proton transfers and the resulting steric effects that are associated with reorganization.
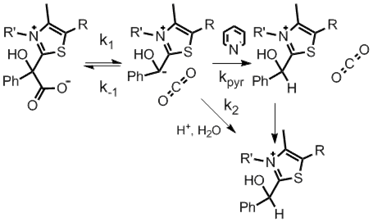
Hydrolytic Route
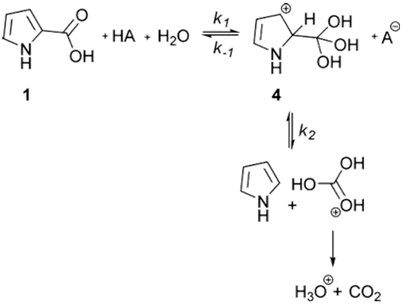
We have also found that there is a distinct alternative to the loss of CO2: acid-catalyzed addition of water to the carboxyl group and formation of carbonic acid. Protonated CO2 is an impossible intermediate yet in some cases reactions proceed in highly acidic solutions. This route is analogous to ester hydrolysis and appears to be accessible in systems that provide adequate leaving groups. We are examining the extent to which this process occurs in the many reactions that are reported to undergo acid catalyzed decarboxylation.
Aminoacyl phosphate monoesters and reactions with t-RNA
These novel reagents are functional analogues of the critical intermediates in ribosomal protein synthesis. They can be used for protein modification, peptide synthesis and, potentially, to produce aminoacyl tRNA. We have found that lanthanide ions are particularly effective catalysts for the addition of oxygen nucleophiles to acyl phosphate monoesters in general. Our research is aimed at extending and improving the approach.
Reactions are selectively catalyzed by lanthanides (recognition of diol in water)
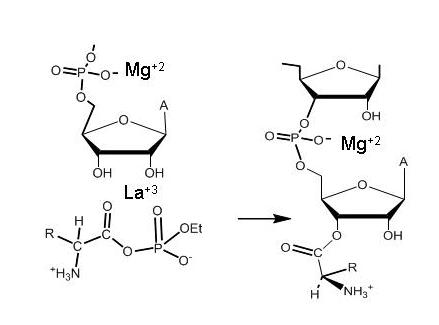
Aminoacylation of RNA
Learn more about this with a slide presentation: Click here
Biomimetic peptide coupling
The aminoacyl phosphates are also efficient reagents for producing peptide bonds in water by reaction with amino acid esters. This mimics the non-ribosomal formation of peptides. We are expanding this as a method to produce peptides under conditions that are amenable to biological catalysts.
